Abstract
【Introduction】There is no standard treatment for idiopathic multicentric Castleman disease (IMCD) in countries or regions where siltuximab is inaccessible. This study aims to explore the efficacy and safety of bortezomib combined with cyclophosphamide and dexamethasone (BCD) in the treatment of newly-diagnosed IMCD.
【Methods】 A total of 30 newly-diagnosed IMCD patients in our hospital from December 2018 to March 2021 were enrolled in the study. BCD regimen (bortezomib 1.3 mg/㎡ weekly subcutaneously, oral cyclophosphamide 300 mg/㎡ weekly, oral dexamethasone 40 mg weekly for a 28-d cycle) was administered for 9 cycles; After 9 cycles of BCD treatment, BD regimen (bortezomib 1.3 mg/㎡ every two weeks subcutaneously, dexamethasone 20 mg every two weeks was used as maintenance for one year. The primary endpoint of the study was the overall response at 24 weeks. Treatment responses were evaluated according to the CDCN criteria.
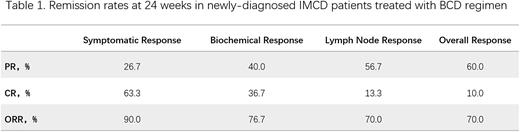
【Results】The median age of 30 patients was 48 (22-77) years old, and the male to female ratio was 1.5:1, including 12 cases of non-severe IMCD NOS (40%), 11 cases of severe IMCD NOS (36.7%) and 7 cases of TAFRO syndrome (23.3%). The overall response rate (CR or PR) at 24 weeks of treatment was 70.0%, with an overall complete remission rate (CR) of 10.0% and an overall partial remission rate (PR) of 60.0%. The symptomatic, biochemical and lymph node remission rate at 24 weeks was 90.0%, 76.7% and 70.0%, respectively. The overall remission rate at 24 weeks was 66.7% in severe patients and 75.0% in non-severe patients. The median follow-up time was 26.5 months (range,2-46 months). The median time to next-line treatment (TTNT) was 37 months (range, 1-46 months). The median TTNT in severe patients was not reached, and the median TTNT in non-severe patients was 37 months (10-40 months) (HR 1.26, 95% CI 0.37-4.27). In terms of safety, no grade 3 or above adverse reactions or treatment-related deaths occurred.
【Conclusions】BCD regimen may be an effective and safe treatment for patients with newly-diagnosed IMCD, especially for patients in severe group.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal